
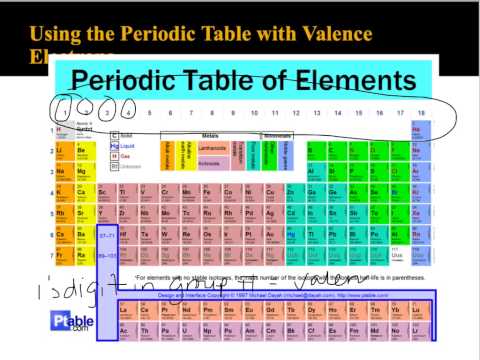
Okay, so for chlorine, we see that we have our seven valence electrons in the outer shell, with all the remaining electrons serving as our inner core electrons.\): Periodic table by Dmitri Mendeleev, 1871. Atomic number The number of protons in an atom. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). And in this third show we have our seven valence electrons, The remaining 10 electrons are in shells too, And one they total up to a total of electrons. Elements are organised into blocks by the orbital type in which the outer electrons are found. And you can see here with this representation of the chlorine atom We have here are 3rd shell, so and equals three. So that would mean out of the 17 total electrons.
The group number helps indicate the number of. Chlorine has an atomic number of 17 When it is neutral it has 17 protons. The number of valence electrons for each element actually varies directly with the group the element is in.
Periodic table valence electrons plus#
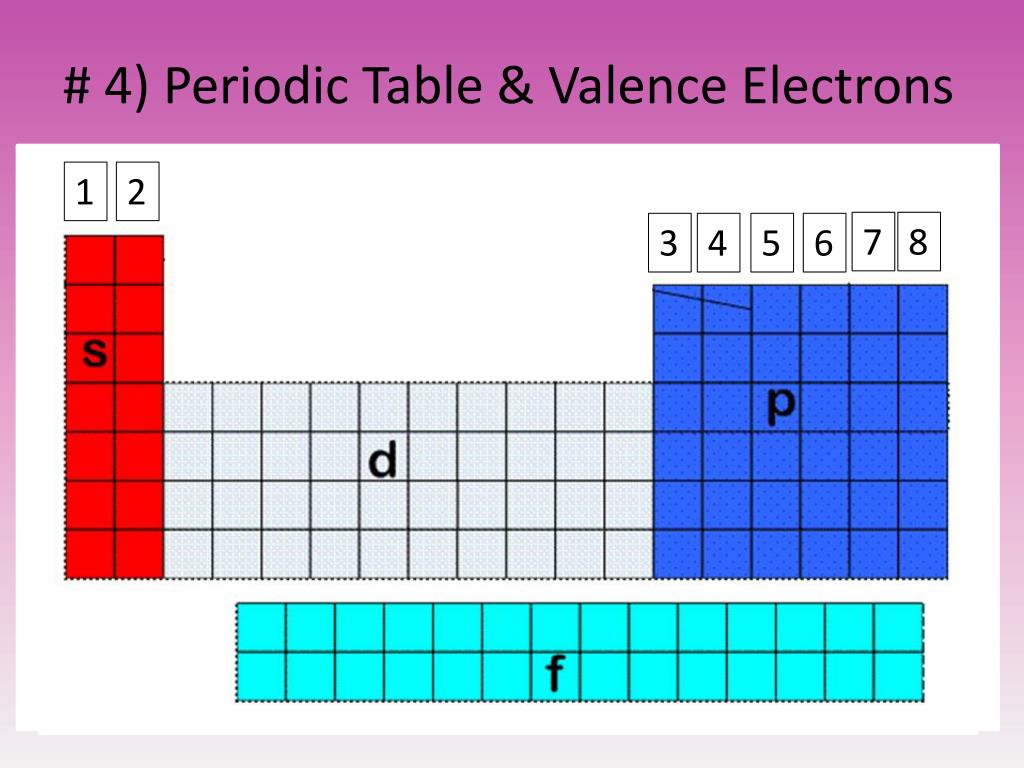
So you're gonna say total electrons Which is connected to your atomic number for neutral element equals your valence electrons plus your inner core electrons. We're going to say here that your total number of electrons. Valence electrons are the electrons of an atom that can participate in chemical bonding, for example, for H and He the 1s electrons, for Li through Ne the. Valence electrons are the electrons located in the outermost shell or energy level of an atom that are involved in chemical bonding. These are named after the orbitals, so there. The number of valence electrons depends on the octet rule. Elements within the same group share the same number of valence electrons. Atoms in a period have the same number of electron shells. So these are the remaining electrons that are not valence electrons. The periodic table can be broken up into different blocks based on which orbitals their valence electrons occupy. An element period is a horizontal row on the periodic table.
Periodic table valence electrons how to#
Elements in the same group on the periodic table have the same number of valence electrons. Every atoms goal is to have a complete valence shell with 8 electrons (or, in Hydrogens case, 2). 158K views 5 years ago The Periodic Table Valence Electron Basics Learn how to use the periodic table in order to determine the number of valence electrons. A gap in ionization energies could tell us how many valence electrons an element has. According to the periodic table, oxygen is in group 6 therefore, it has 6 valence electrons in its outer shell. Valence electrons are found in the s and p orbital of the outermost shell. Now, besides the valence electrons, all the remaining electrons are called your inner core electrons. Valence electrons are the outermost electrons in an atom. Chemical bonding is the result of the interactions between two or more atoms valence electrons.
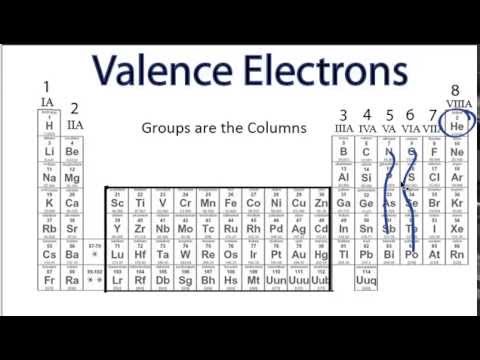
Now these valence electrons are the outer shell electrons involved in forming chemical bonds. Valence electrons are the only electrons in an atom which will interact with other atoms. Now for main group elements were going to say that the number of valence electrons that they possess equals their group number.


 0 kommentar(er)
0 kommentar(er)
